How do nanocrystals form? – Publication in JACS by CRC 1411 researchers
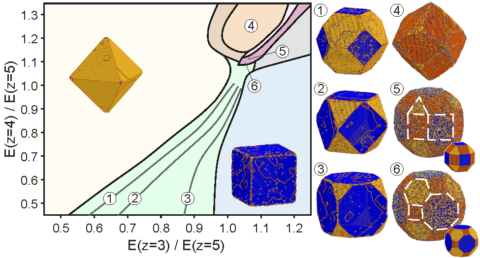
Scientists have long known that the shape of tiny crystals (nanocrystals) can affect their properties, such as how they interact with light or how well they function as catalysts. However, precisely understanding how these shapes form has been challenging, since many factors are involved: temperature, chemicals in the solution, and even the arrangement of atoms at the crystal’s edges. In this study, the researchers introduced a theoretical and computational approach that focuses on the critical spots where atoms attach as the crystal grows. By examining the energy required to add atoms at these sites, they explain why certain shapes, including cubes, octahedra, or other symmetrical forms, often appear.
Beyond explaining why various shapes emerge, this method can guide experimental design. It enables scientists to predict which conditions favor different morphologies and to control crystal growth toward specific outcomes. The study shows that the first atom to grow on the surface, referred to as the adatom, dominates the process by creating temporary pathways that lead to particular shapes. This new understanding not only clarifies how nanocrystals form but also suggests ways to refine their shapes for targeted uses in technology and medicine.
Read about this work here:
Carlos L. Bassani, Michael Engel
Kinetically Trapped Nanocrystals with Symmetry-Preserving Shapes
Journal of the American Chemical Society 147, 9487-9495 (2025)
